Staying on top of the game
Learn how TE supported a pioneer in the PCI market seeking to refresh its product family for PTCA interventions responding to changing dynamics in the market.
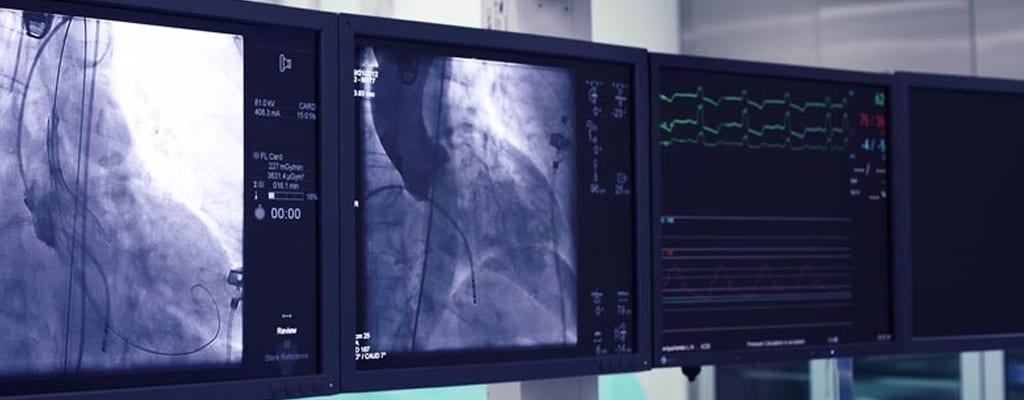
Our customer, a pioneer in the PCI market, sought to refresh its product family for PTCA interventions responding to changing dynamics in the market. Recognizing TE's undisputed leadership in PTCA catheter design and manufacturing, a partnership commenced.The Design team were presented with 3 challenges: to develop a high performance PTCA catheter incorporating novel balloon materials; reduce the overall cost of manufacture, and realize regulatory approval in a narrow timeframe.



Matching TE's PTCA catheter platform or ready-made catheter chassis with the customer's innovative balloon material, the basis for the new design was quickly in-place. Shifting focus to a cost effective 'design for manufacture', a 50% reduction in unit cost was achieved. Taking full regulatory responsibility for the product, our Design team achieved CE mark 16 months from project start with FDA clearance 4 months later, beating the customer's timeline expectations.Designed in California and manufactured in Singapore, TE continue to manage this PTCA balloon catheter product as the PTCA market evolves.
