
Vertebrostenting: beyond the vasculature
Learn how TE partnered with an emerging company to design an innovative yet simple to use vertebrostenting system, exceeding the customer's high expectations.
Deployment of stents in the vasculature is complex but established. Our customer, an emerging company, conceived stent deployment for damaged vertebrae. By combining Minimally Invasive delivery techniques with a therapeutic dual stent & bone cement solution, the clinical vision is to treat compression fractures in the world's growing osteoporosis patient population of 1.4 million new cases per annum. Pushing the boundaries of medical device design, we assembled a cross functional team to realize the vision of our customer, a 'first of its kind' highly complex product. Working through a suite of rotational and non-rotational design concepts for the base outer sheath and inner pushrod system, an innovative yet simple to use vertebrostenting system was devised, exceeding the customer's high expectations.
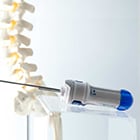


Key challenges encountered and resolved during the design engagement were:
- Mounting and deployment of the stent at the distal end of the system to ensure that the stent could withstand force and remain secure in position.
- Ensuring all joints were capable of surviving the rigours of the procedure and the injection force of the cement.
- Maintaining flexibility in the distal portion of the deployment system while simultaneously accommodating the mechanical forces applied during the unsheathing process.
- Ergonomic and human factors design to incorporate handle and deployment buttons that are simple to use and conceal the complexity within
A critical aspect of the project was the flexibility shown by the TE design team to deliver on 6 monthly milestones throughout the development programme, supporting the initiatives being made by the customer in driving the path to commercialisation. Having demonstrated clinical success, the original development company was subsequently acquired by the world's largest privately held spinal implant manufacturer.



