IVD Microfluidic Reagent Blister Pack Manufacturing
If you’re aiming to develop a consumable microfluidic lab-on-a-chip version of your assay, it will probably incorporate reagent blister pack technology. We have been at the forefront of designing and manufacturing blister packaging for IVD consumables for 20+ years and are ready to tackle your project.
We Design for Manufacturability
In the early stages of development, performance and reliability are the key areas of focus. Yet, based on decades of experience, we know that once a design has been proven, the emphasis quickly shifts to how cost-effectively the cartridge can be manufactured. Because we design and manufacture IVD consumable cartridges and IVD reagent blister packs, we understand the manufacturing and assembly steps needed to deliver stable, consistent and cost-effective microfluidic consumables.
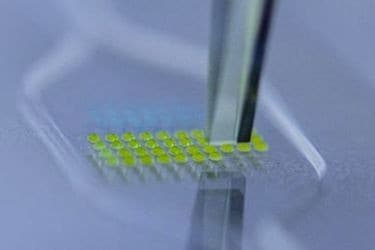
Reagent Blister Pack Configurations
- Dome Blisters
- Custom Geometry Reagent Blisters
- Variable Size Blister Arrays
- Multiple Liquid Blisters
Moving from Prototype to Gap Manufacturing to High Volume Production
Successfully transferring your newly designed technology from prototype to mass manufacturing requires an in-depth knowledge of materials science, blister foils and forming and microfluidic manufacturing techniques. Our team has extensive experience designing IVD microfluidic consumable cartridges incorporating on-card reagent blister pack technology.
- Blister production capability from <50uL to >3mL
- Off-the-Shelf blisters for prototyping in 50/100/200/500/1000uL capacities
- Rapid custom blister design for specific volume and delivery profiles
- Varied geometry, asymmetrical blister shapes to maximize card space
- Multiple opening and dispense methodologies: built in opener, spike opening
- Can provide instrument actuator designs or disposable actuator built into card
- Guidance for device actuation or manual actuation
- Blister labelling for efficient handling and foolproof assembly
- Single blisters capable of multiple buffer dispensing cycles, plus other uses
Our capabilities are not limited to consumable design and manufacturing. We have an entire team specializing in usability testing for point of care diagnostic devices, and a full clinical research team that’s ready to perform necessary studies to validate your design and get it through regulatory approval. Companies often lose precious time shifting between partners, but we have all of these capabilities in-house. As such, we can often get your device to market faster and save you money overall.
Customized and High-Volume Automated Manufacturing Ensures Blister Pack Availability and Consistency
As an ISO-13485 certified development and manufacturing firm, we have the experienced team, equipment and facilities to transition your IVD consumable device from 5 thousand to 5 million to 50 million , all within our Quality Management System or yours. Our sophisticated fill and seal technology ensures reliable, accurate and consistent dispense.
We can control the environment during filling and sealing, even to producing sealed blisters under vacuum or inert atmospheres. And, for methodologies sensitive to DNA, RNA or RNase contamination, we maintain a production suite with mitigation for these and other biologic contaminants. For IVD card assembly with blisters that must be within a particulate controlled environment, we have a Class 10,000 clean room within the production suite.