
A to Z design partnership
Learn how TE was able to provide mechanical development & manufacturing support to a Top 3 company in the Atrial Fibrillation (AF) devices market.
Atrial Fibrillation (AF) is one of the fastest growing device markets in cardiology. There are an estimated 12.5 million symptomatic AF patients in the world, many of whom are not being treated effectively.
Our customer, a Top 3 company in the AF device market, wanted to develop a next generation access sheath to introduce its mapping and ablation catheters to the left side of the heart. In this burgeoning market, the customer's own design resources were already fully occupied in developing its therapeutic device portfolio - that is when TE stepped in. Providing the skills and proven infrastructure to deliver a complete solution, our team was the perfect fit to bring this complex steerable access sheath from design & approval to commercial manufacture on behalf of the customer.

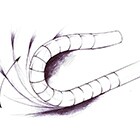

Initiating the project with the customer's clinical feedback and a list of desirable needs, TE completed product development, design verification, validation, ramp for commercialization and secured product approval. In less than 2 years, the customer was successfully performing commercial clinical procedures in multiple regulatory geographies worldwide. TE continues to manufacture this complex device for the customer today.
Along with meeting the core clinical needs of the product, the design team delivered a truly differentiated solution incorporating innovations such as multiple targeted transition zones, novel asymmetric sheath articulation and an industry first in an independently rotating side-arm attachment to the hemostasis valve.



