
Accelarating TAVI Delivery
Learn how TE partnered with a Structural Heart pioneer to become one of the first three companies to achieve approval of a 1st generation TAVI system.
An estimated 5% of the world's global 65+ year old population at risk of developing valvular heart disease. Minimally Invasive approaches to Structural Heart therapy are emerging rapidly, including the blockbuster market of TAVI - Transcatheter Aortic Valve Intervention. In this procedure, using catheter based techniques, a bioprosthetic replacement valve is navigated to the native diseased valve and deployed in its place. Replacing the native valve is not the only clinical challenge in TAVI - Structural Heart patients typically present with highly diffuse, calcified vascular disease which complicates transcatheter access and delivery. Coupled with the large diameter delivery systems synonymous with TAVI, initial device "introduction" underlies overall procedural success. Our client was seeking to become one of the first 3 companies to achieve approval of a 1st generation TAVI system. Focusing on the highly complex therapeutic aspect, the client also recognized the necessity of a compatible introducer sheath.
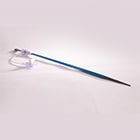
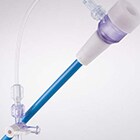
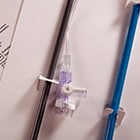
To ensure no delay in their core TAVI programme and given the company's limited resources, the customer sought a suitable partner to design, develop and manufacture their custom introducer sheath and fulfil the role of legal manufacturer. In a quick 9 month timeframe, the Design team successfully delivered a CE marked product, fully compatible with the customer's TAVI system. The 510k cleared product was delivered by blending TE's core expertise in sheath design and manufacture, with its proven design process and regulatory competencies. Technically, the custom introducer sheath optimized stiffness and flexibility to facilitate seamless introduction of large bore devices. A custom cut pattern was developed for the critical hemostasis seal element to ensure excellent sealing as the dilator and valve are introduced. This pioneering company was subsequently acquired by a leading medical device company. TE continues to fulfil the role of legal manufacturer for this product.



