Material selection in the medical devices industry can be a complex process. TE’s experts have a proactive strategy to evaluate evolving regulations (including PFAS) and their potential impact to current and future medical device design. This strategy, includes understanding what needs to change if regulations ban certain materials, identifying alternative materials and supply channels, and their presumed impact on availability, cost, efficacy, feasibility, manufacturing processes and equipment, and safety to help ensure the device is designed to achieve optimum quality and performance. In the webinar, TE will discuss the process of evaluating and selecting new materials to design and manufacture proximal shafts that optimizes performance, quality and scaling, while considering potential regulatory concerns.
Product Group
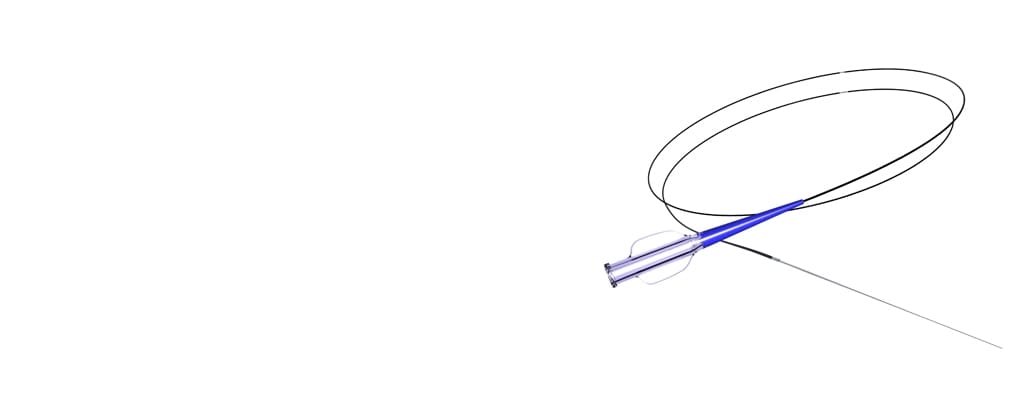
Metal Shafts & Hypotubes
TE is a leading provider of high-precision metal shafts and hypotubes that are utilized in the development of minimally invasive medical devices solutions, including structural heart intervention, electrophysiology mapping and ablation catheters, and surgical robotics.
Connect to a strong legacy of innovation. With a strong foundation of expertise stemming from our acquisitions of MicroGroup and Creganna Medical, we are well-equipped to assist you from prototyping to large-scale production. We offer an extensive inventory of hypotubes that can be tailored to meet your specific requirements, enabling you to enhance performance, reduce lead times, and optimize costs in the development of your next-generation medical devices.
Design Considerations
Achieving optimal clinical performance for your hypotube begins with an in-depth understanding of the performance requirements for your product application. Attaining the best performance in one characteristic can often directly affect other characteristics. We have the engineering and manufacturing experience and expertise to support you in making these critical design decisions.
Hypotube Design Considerations for Optimal Performance
- Transition – Custom engineered transitions to optimize product performance using process technologies and features such as laser spiral cutting, a skive feature or corewire weld.
- Trackability – A wide variety of coating technologies to maximize trackability, including discrete polymer overjacketing.
- Push and Torque – Optimization of these key performance characteristics ensures consistent and reliable location of the treatment site.
- Kink Performance – We offer both design advice and PoleVault™ hypotube product to optimize kink performance.
- Compression Resistance – Requirements should be defined in specific terms prior to prototype development e.g., a 1,000 mm shaft must exhibit less than 1% compression where a 4lb force is applied.
- Flexibility – Flexibility requirements can be specified in terms of the bend radius that must be achieved e.g., the shaft must be capable of bending around a 30 mm diameter.
Materials & Specifications
Partnering with us enables you to access a wide range of materials, engineering and manufacturing expertise, backed by a global footprint. Delivering the optimal hypotube for clinical performance and usability requires an advanced range of technical solutions.
Technologies
- Distal shaft technologies - The transition or finish of the distal end of the metal shaft is of vital importance to the overall metal shaft design, as it plays a pivotal role in overall clinical performance. Technology features of distal transition zones range from simple burr-free cuts to more complex spiral, skive, slot, crimp, taper, or flare designs. Additional features can be added to the distal transition zone such as corewires, coils, or custom micro-machined components to add additional flexibility or smooth the transition to the distal assembly.
- Intermediate shaft design - Working with you, we can develop the optimal intermediate shaft design for your delivery device. We assist with critical design decisions relating to material choices, properties, and design alternatives. Advanced laser processing techniques can dramatically transform a metal shaft to increase flexibility and torque response. Appropriate femoral, radial, or brachial markers can also be applied. We also provide a range of coating solutions along the intermediate shaft from simple FEP coatings to polymer overjackets that combine the strength of a metal shaft with the flexibility of an outer polymer jacket. Your choice of coating solutions will impact catheter shaft lubricity and overall device trackability.
- Proximal Shaft Design - Design choices for the proximal shaft will affect the overall usability of the delivery device or catheter. The most common and simple solutions include luers or hubs which facilitate basic access to the catheter lumen for the injection of fluids or drugs or the introduction of guidewires and sheaths. These components can be combined with an appropriate strain relief mechanism and over-molded to create a finished metal catheter shaft sub-assembly. More advanced proximal designs include complex handles and deployment mechanisms that integrate mechanical, electronic, or diagnostic mechanisms – all of which we can design and develop for you.
Materials
| Materials | Specifications | Surface Solutions |
|---|---|---|
| Medical grade stainless steel - 304, 304L, 177 |
|
Polymer overjacket, Standard Ultrathin PEBAX®, Nylon, Polyimide, FEP, Polyethethylene |
| PoleVault - Superior kink resistance | Customer specified;
|
Marker bands;
|
| Javelin - Superior push | Customer specified;
|
Marker bands;
|
Transition Solutions
| TRANSITION SOLUTIONS |
COMPONENTS | PERFORMANCE |
|---|---|---|
| Burr free | Microwelded components |
Laser profiled cut geometries for;
|
| Skive | Microwelded components | |
| Wire Weld | Insert molded thermoplastic components | |
| Flare | Insert molded thermoplastic components | |
| Slot & Crimp | Insert molded thermoplastic components | |
| Ground | Insert molded thermoplastic components |
Platforms & Capabilities
Product Platforms
Innovative Shaft and Hypotube Technology Platforms
TE has existing solutions for metal shafts and hypotubes. These products may provide the solution you’re seeking or a starting point that can be customized to meet your specifications.
PROx Gen2 hypotube range: High-performance proximal catheter shaft subassembly
The PROx Gen2 range is the latest innovation and evolution of the gold-standard PROx high-performance proximal catheter shaft subassembly. PROx Gen2 uses very limited PFAS materials or none, dependent product version, which limits the amounts of harmful substances that are produced and discharged back into the earth. It retains the premium Rx catheter design features of the PROx range, including TE’s unique material - Polevault - and is manufactured in a high-yield, robotic, semi- or fully automated environment.
PoleVault™: The gold standard hypotube
A hypotube solution with up to 40% kink resistance. PoleVault™ hypotubes are specifically designed to optimize catheter performance, thus increasing physician confidence in your product, reducing patient risk, and maximizing catheter revenue.
MICROFLEX™: A highly flexible micro-metal tube >0.013”
A micro hypotube specifically developed for minimally invasive treatment of the body’s smallest vasculature, including interventional neuroradiology, and available in dimensions of 1f or less. The single-piece construction provides the physician with a consistent user experience. It is completely customizable, which presents numerous design possibilities. The single-piece design also maximizes end-product margins by decreasing component and assembly costs.
Capabilities
Customization, Material Science, and Prototyping Capabilities
Any hypotube can be customized to your specifications. Whether you use stock materials or want something completely unique, we have what it takes to get the job done. To aid the development of custom materials and your next-generation medical device, we have dedicated engineering and manufacturing resources.
Material Science Laboratory
Our material science and prototyping laboratory is equipped with advanced characterization equipment and staffed by a team of experienced metallurgist and polymer material scientists. They investigate, develop, and work to optimize the microstructure of medical device materials.
PROPELUS™ Prototype Center
Located in Galway, Ireland, recognized internationally as one of the world’s most innovative, integrated, and globalized MedTech hubs. Our goal is simple: provide our customers with high-quality prototypes as quickly as possible. Learn More.
Manufacturing Locations
Technical Resources
The minimally invasive medical device industry is a dynamic industry, evolving to deliver smaller and more complex devices to treat all kinds of medical issues. Precision manufacturing of laser processed components tailored for these dynamic needs is becoming more critical. In this Device Talks Tuesdays webinar, the TE experts discuss how you can harness cutting-edge laser technology, both cutting and welding, to complete your new delivery and access device or enhance your existing device.
 e
e
 e
e

 e
e
 e
e
 e
e
 e
e
 e
e
 e
e
 e
e
 e
e
 e
e
 e
e
