Our vision is to be your partner of choice, helping build medical devices that save lives. We’re focused on reliability and durability, delivering value through a relentless pursuit of the standards that matter most to you. With a commitment to progress and an extensive product range, we enable medical device OEMs, large and small, to transform ideas into reality.
If our standard component solution don’t meet your needs, our engineering team can collaborate with you to develop custom solutions. With global manufacturing facilities, engineering expertise, we are equipped to design, build, optimize and manufacture your unique solution – from prototype to high-volume manufacturing.
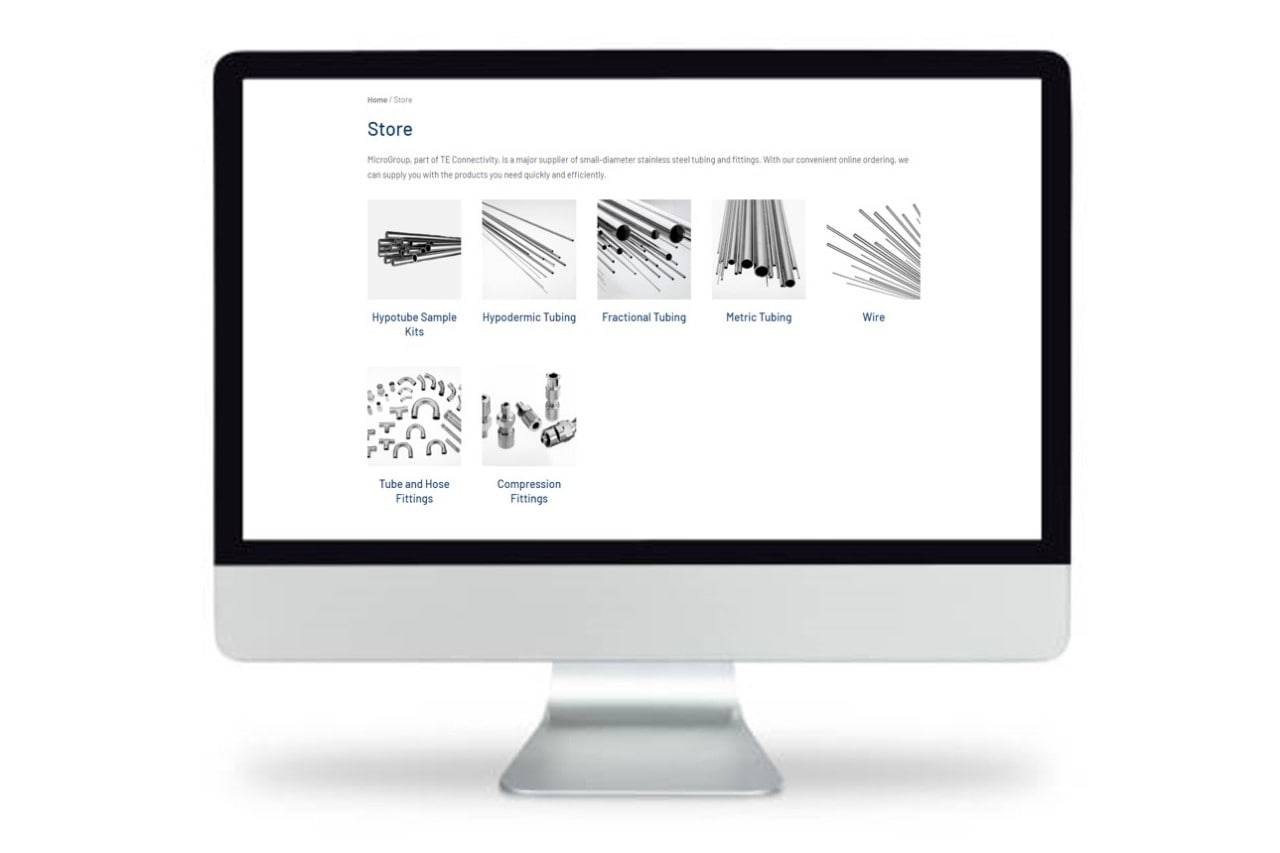
Medical Product & Components Offerings

From design, human factors engineering, rapid prototyping, clinical research to volume manufacturing, we understand the product commercialization cycle and offer a number of skilled technical teams who can support you. With TE, you can bring your concept through approval, prototype, pilot manufacturing, and ramp.
Explore Services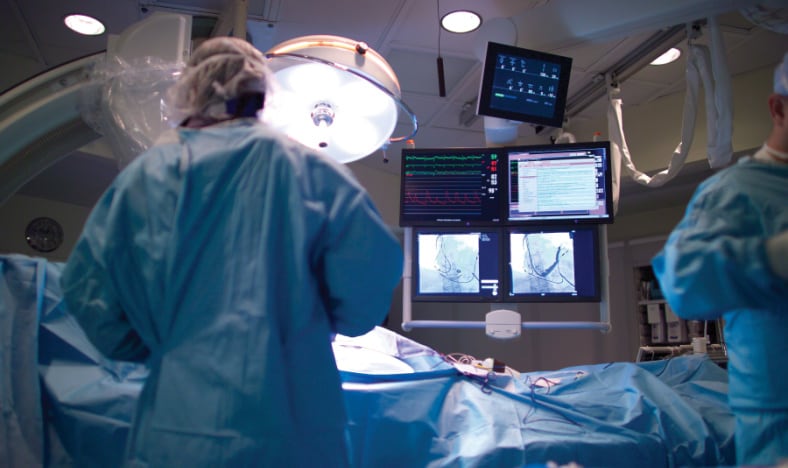
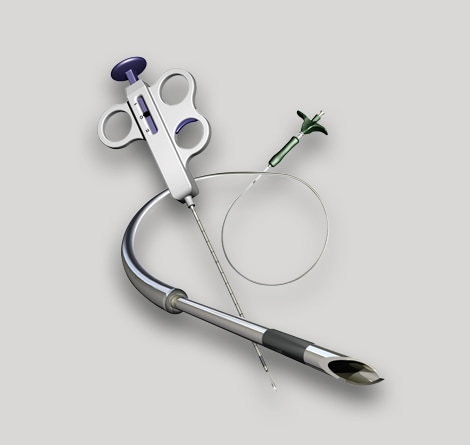
We provide top tier engineering and manufacturing expertise to help you innovate and manufacture key components and sub-assemblies for various medical specialties: interventional cardiology, surgery, imaging, and others.
Explore our solutions for your medical deviceConnect to a Higher Standard of Customer Dedication
TE Connectivity is a partner of choice to the world’s leading medical technology brands. We provide top-tier engineering expertise and manufacturing excellence in the development of optimally engineered components and value-added sub-assemblies to support the production of life-saving medical devices used in interventional cardiology, surgery, imaging, and other medical specialties.






 e
e
 e
e
 e
e
 e
e
 e
e
 e
e
 e
e
 e
e
 e
e

 e
e




 e
e
 e
e
 e
e
 e
e

 e
e
 e
e
 e
e
 e
e
 e
e