EMI Shielding Design Considerations at the Heart of Medical Equipment Compliance and Performance
Download WhitepaperAs technology evolves, EMI shielding becomes increasingly important. EMI shielding, as a key component of engineering design, should be considered at all levels of design from the PCB layout to the enclosure. Engineers are faced with an array of shielding options to suit their needs at each design stage.
Multiple circuits and components in electronic devices emit electromagnetic fields (which can be conducted or radiated) of varying strengths, causing a potential for interference that can degrade or disrupt performance. Circuits should generally be designed so that they don’t add to or become disrupted by the interference. With the demand for miniaturization rising, design engineers often create compact circuits that do not generate EMI and RFI and that function properly while surrounded by internal and external sources of EMI and RFI. This makes it crucial for some designers to perform EMI/RFI measurements and software simulations during different stages of the design process, measuring EMI and RFI emissions across a wide frequency range. Failing an electromagnetic compatibility (EMC) compliance test can be costly—in time and money. Designers often spend valuable time reworking circuit layouts and without proper testing and measuring during the redesign process, could potentially fail another EMC test.
There are many types of EMI Shielding capable to protect all the critical components and increase performance, reliability, and value to medical professionals. With the current trends and demands on the medical industry, TE Connectivity’s EMI shielding technology will enable your design to optimize performance and compliance.
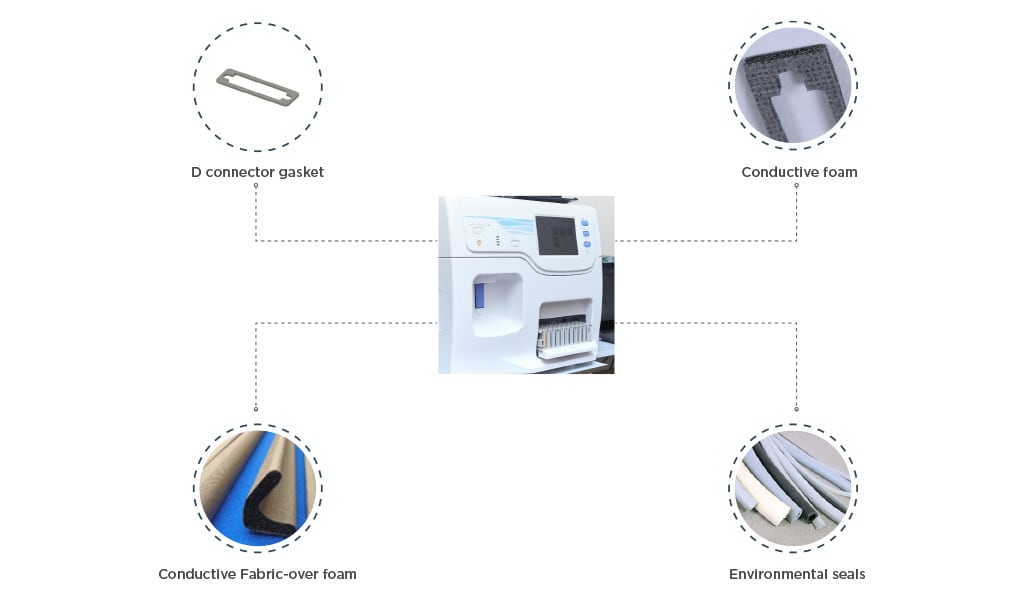
EMI Shielding in electronic devices and equipment is the use of manufacturing techniques and materials to protect signals from being disrupted by external electromagnetic signals as well as preventing generated signals from interfering with surrounding components.








